Exotic Crystal Chemistry at Extremes
Exotic Crystal Chemistry at Extremes Heading link
Although most of chemistry as we experience it commonly takes at close to 1 atmosphere, the range of pressure in the universe actually covers an incredible range, from the near-zero pressures of interstellar space to the centers of neutron stars. With advances in the technology of high pressure devices, and the development of new analytical methods for investigating matter at high pressures, the extreme conditions research community continues to uncover new examples of the surprising effects of applied pressures far away from our familiar 1 atmosphere environment.
In a new review article, CDAC Postdoc Katie Hilleke and CDAC Partner Eva Zurek highlight some of the surprising effects of high pressure, typically up to the 200-300 gigapscal (2-3 million atmospheres) range, on structure and bonding in a wide variety of materials. Starting with the ways in which pressure modifies atomic energy levels on compression, the authors proceed to discuss the response of crystals to applied pressure, both in terms of overall crystallographic structure and how this is related to changes in the overall electronic structure of the solid.
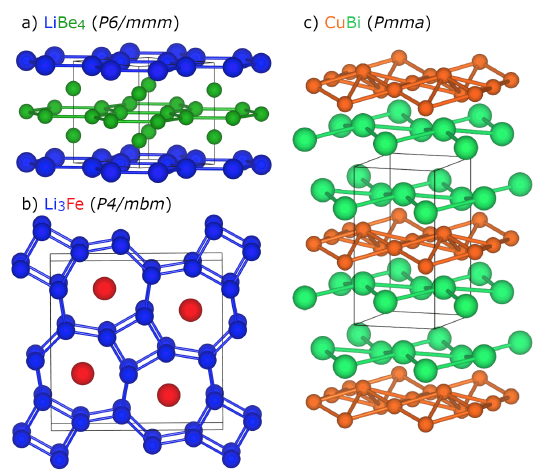
With this background, the authors describe a variety of materials where applied pressure produces truly novel behavior, including high pressure electrides, solid compounds that contain electrons that play the role of negatively charged ions, and noble gas compounds such as Na2He and related materials that only form at pressures sufficient to allow the “inert” He atom to react. Unexpected chemical compositions, such as those shown in Fig. 1 that are predicted or observed to become stable only at high pressures, and unusual bonding motifs that emerge in elemental materials such as boron, attest to the rich array of structure and bonding systematics yet to be explored and developed with emerging computational and experimental methods. Hilleke and Zurek also highlight the surprising effects of pressure on superconductivity in hydrogen and other elements, as well as hydrides in both clathrate-like and covalent systems.
[1] Feng, J., R. G. Hennig, N. W. Ashcroft, and R. Hoffmann, Emergent reduction of electronic state dimension in dense ordered Li-Be alloys. Nature 451, 445-448 (2008).
[2] Zhou, Y., Q. Xu, C. Zhu, Q. Li, H. Liu, H. Wang, and J. S. Tse, Predicted lithium-iron compounds under high pressure. RSC Advances 6, 66721-66728 (2016).
[3] Guo, K., L. Akselrud, M. Bobnar, U. Burkhardt, M. Schmidt, J.-T Zhao, U. Schwarz, and Y. Grin, Weak interactions under pressure: hp-CuBi and its analogues. Angewandte Chemie International Edition 56, 5620-5624 (2017).
[4] Clarke, S., M. Amsler, J. P. S. Walsh, T. Yu, Y. Wang, Y. Meng, S. Jacobsen, C. Wolverton, and D. E. Freedman, Creating binary Cu-Bi compounds via high-pressure synthesis: A combined experimental and theoretical study. Chemistry of Materials 29, 5276-5285 (2017).
Hilleke, K. P. and E. Zurek, Crystal chemistry at high pressure. Reference Module in Chemistry, Molecular Sciences and Chemical Engineering: Comprehensive Inorganic Chemistry III ; DOI:10.1016/B978-0-12-823144-9.00170-9. Elsevier, 2022.