Chemical Interactions That Govern the Structures of Metals
Chemical Interactions That Govern the Structures of Metals Heading link
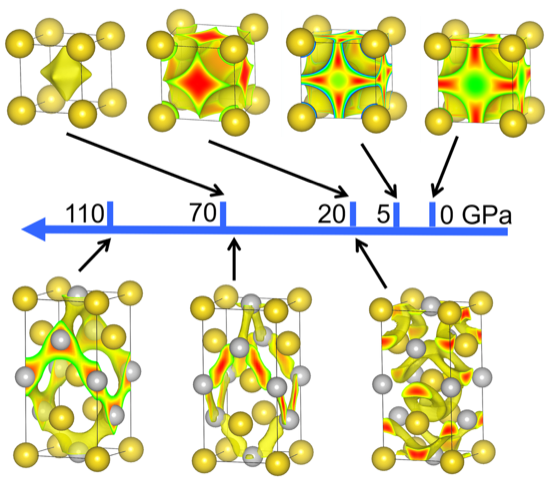
What determines the structures of simple metals has been a subject of a longstanding debate among researchers, and findings from a new study appearing in the Proceedings of the National Academy of Sciences add new information to consider in the discussion.
By analyzing the electronic properties of metals in various lattices over a broad range of sizes and geometries, CDAC Director Russell Hemley and collaborators shifted from a “physics” band-structure point of view to a “chemical” perspective to find highly complex structures that emerge at high pressures in these materials. This has led to a new theory that can explain why a particular structure is favored for almost all metals in the periodic table.
“This theory is based on our finding that the electrons in many metals occupy what we call quasi-atom orbitals — the local quantum orbitals centered at the voids between the atoms. In large measure, it is the chemical interactions between localized electrons that control the structure of the metals,” Hemley said.
The result contrasts with the traditional view that structures and other properties are determined by the extended electronic states, which behave close to what is known as a free electron gas. However, the structure preference of classes of metals across the periodic table cannot be explained by this “physics,” or band structure point of view.
One reason the research team is particularly excited about this work is that the idea originated from the need for a simple “chemical’ explanation for the existence of high-pressure electrides, a surprising phenomenon that includes for example the fact that some alkali metals become transparent insulators under pressure. The findings have implications for the behavior of chemically complex materials, including alloys, intermetallics, hydrides, ionic compounds and two-dimensional materials.
“This is an example of how the study of matter under extreme conditions can inform us about chemistry and materials under normal, or more familiar conditions. Thus studies of materials at high pressure can reveal chemical features that might be otherwise neglected,” Hemley said.
Sun, Y., L. Zhao, C. J. Pickard, R. J. Hemley, Y. Zheng, and M. Miao, Chemical interactions that govern the structures of metals. Proceedings of the National Academy of Sciences USA, 120, e2218405120 (2023).