4. Extreme Chemistry
4. Extreme Chemistry
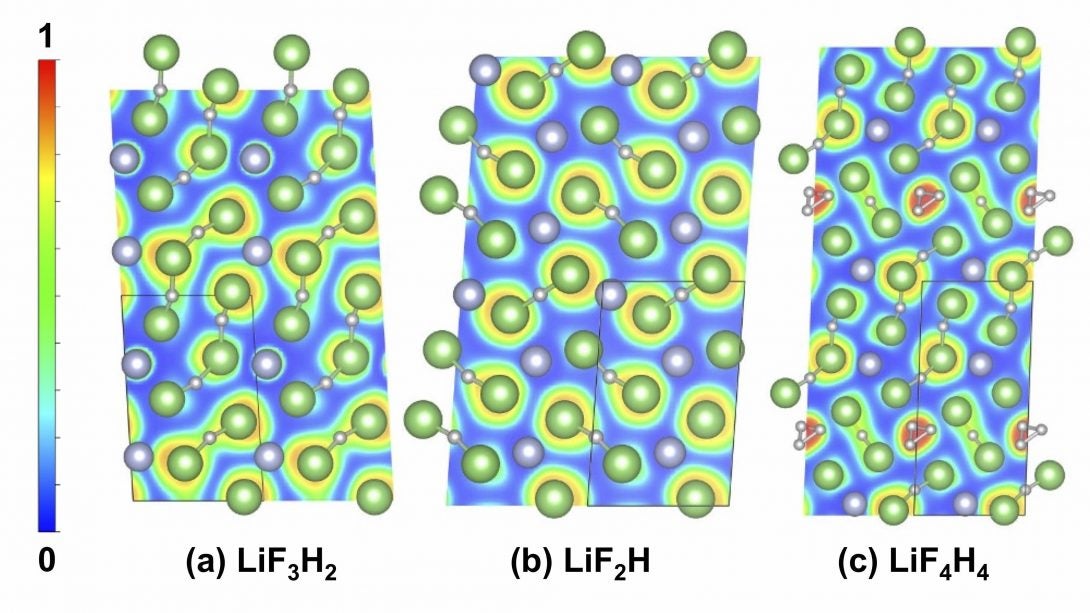
Extreme Chemistry explores the frontier of chemical bonding, structure and related properties of the elements at extreme conditions, including the ‘New Periodic Table’ under pressure. Two collaborations involving theoretical studies, coordinated by CDAC Academic Partner Eva Zurek (Buffalo) highlight work in this thrust area. In joint work between groups at Buffalo, UIC, and LLNL and led by former CDAC student Tiange Bi, new chemistry has been uncovered in the Li-F-H ternary system, which is important in particular for dynamic compression experiments involving lithium fluoride and hydrogen [1]. CDAC postdoc Katie Hilleke has led a collaboration between Buffalo, LLNL, and LLE to identify novel structures and bonding in boron at megabar pressures [2]. Recent efforts in Extreme Chemistry includes synchrotron IR measurements at FIS /NSLS-II on the synthesis of atomically thin hexagonal diamond with compression, led in part by former CDAC partner Wendy Mao (Stanford) [3]. Extreme states of water have also been investigated at FIS, and have included studies of H2O ice at megabar pressures using synchrotron far IR spectroscopy at FIS.
[1] T. Bi et al., J. Chem. Phys. 154, 124709 (2021); [2] K. P. Hilleke et al., Phys. Rev. Materials 5, 053605 (2021); [3] F. Ke et al., Nano Lett. 20, 5916-5921 (2020).